Artificial Urinary Sphincter
Artificial urinary sphincter is a device that closely simulates the function of a biological urinary sphincter.
The artificial urinary sphincter has been around since early 1970s and remains the standard of care of many men with severe urinary incontinence (e.g. wears more than 3 pads, continuous urine dribble/incontinence) and radiation-related urinary incontinence. Published literature supports this device as highly effective, safe and durable, and is associated with excellent patient satisfaction.
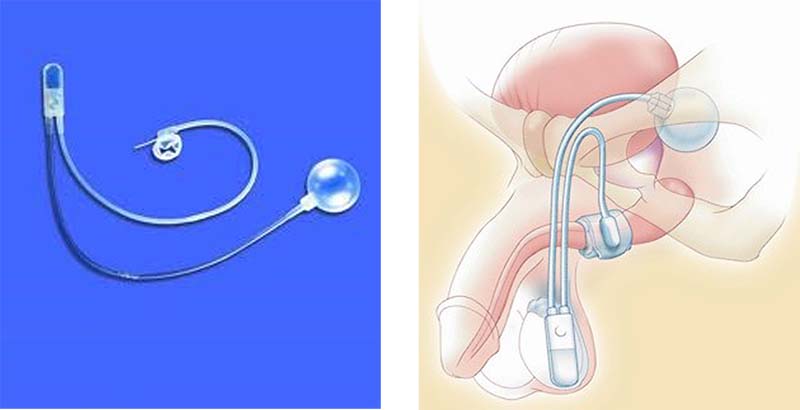
This device consists of 3-parts; an inflatable cuff that surrounds the urethra (or bladder neck in spinal patients and woman), a pressure regulating balloon (filled with saline) that acts as a fluid reservoir, and a control pump (situated in your scrotum or labia). Manual compression of the pump would cause the cuff to deflate, and you can then void. However, within a short time frame (usually 30-60 sec) the cuff will reinflate due to the transfer of fluid from the pressure regulating balloon to the cuff and you become dry again. Sometimes you may need to recycle the device a few times if you have a full bladder to empty.
Professor Chung has published extensively on artificial urinary sphincter surgery (see below) and is considered the leading surgeon expert in Australia and worldwide. He is the only surgeon from Australia and Asia-Pacific invited to serve on the male surgery for urinary incontinence committee at the International Consultation on Incontinence, the peak body for International Continence Society. He has mentored many surgeons over the years including invitation as a surgeon expert at various hospitals and surgical workshops around the world. He is often asked to see complex and salvage cases by other surgeons to restore urinary continence in difficult patients.
What preparation is required?
The artificial urinary sphincter implant is performed under general anaesthesia, and you will need to fast (nothing to eat or drink) for 6 hours prior to your surgery. Regular medications can be taken with a sip of water with the exception of blood thinning agents (such as warfarin, clopidogrel) and non-steroidal anti-inflammatory drugs which need to be stopped for 7-10 days. A mid stream urine (MSU) test is required to ensure the urine is sterile before treatment is undertaken.
What happens in the operating room?
You will meet Professor Chung and your anaesthetist prior to surgery. Your procedure will be performed under general anaesthesia with appropriate anti-microbial cover. The genital area will be shaved in the theatre, and appropriate povidine surgical scrub will be performed to minimise microbial skin colonisation and infection risk.
What are the risks?
Since artificial urinary sphincter implant involves the insertion of a foreign device, strict microbial prophylaxis and safe surgical techniques are paramount. When performed by an expert surgeon, the artificial sphincter implant is a safe procedure with minimal complications.
Common complications involve:
- Pain usually around the surgical sites (inguinal, groin and perineum regions)
- Bruising, bleeding or hematoma
- Dysuria from urinary catheterisation
- Skin (wound) irritation or mild infection
- Persistent or recurrent urinary symptoms, and temporary urinary retention
Potential serious complication include
- Injury to the urethra or bladder during device implant may require the operation to be abandoned due to the high risk of device infection
- Device infection or abscess formation around the device is the most dreaded complication and usually involves the removal of the device
- Device malfunction- commonly due to fluid leak or atrophy of tissue
What to expect afterwards?
You are usually required to stay overnight to receive intravenous antibiotic for 24 hours postoperatively. Your urinary catheter and surgical dressing will be removed the next morning after your surgery. You will remain incontinent of urine for the time until the artificial sphincter can be safely activated. When you are comfortable and passing urine satisfactory, you will be discharged from the hospital with 14 days of oral antibiotics. At home you should rest and avoid strenuous physical exertion for 4-6 weeks.
Follow-up
You may be contacted or have an appointment the following week in order to check on your progress. The recycling of the artificial urinary sphincter occurs at your second follow-up visit with Professor Chung at 4-6 weeks postoperatively. During that visit, you should take simple oral analgesia prior to the appointment as you might experience local pain when the artificial urinary sphincter is activated for the first time.
Some of the key articles published by Professor Chung on artificial urinary sphincter
- Chung E, Liao L, Kim JH, Wang Z, Kitta T, Lin ATL et al. The Asia-Pacific AMS800 artificial urinary sphincter consensus statement. Int J Urol. 2023;30(2):128-138
- Wilson SK, Chung E, Langford B et al. First safety outcomes for Rigicon ContiClassic artificial urinary sphincter. Int J Impot Res 2023. doi 10.1038/s41443-023-00748-8
- Chung E, Wang J, Cartmill R. Is artificial urinary sphincter surgery safe and effective in elderly males aged 70 years and above? Lower Urinary Tract Symptoms. 2022;14(6):416-420
- Chung E. Artificial urinary sphincter surgery in the special populations: neurological, revision, concurrent penile prosthesis and female stress urinary incontinence groups. Asian J Androl. 2020;22(1):45-50
- Chung E. Contemporary surgical devices for male stress urinary incontinence: a review of technological advances in current continence surgery. Trans Androl Urol. 2017;6(Suppl 2):S112-S121
- Gani J, Hennessey DB, Hoag N, Lee D, Chung E. A pilot study of autologous rectus fascial wrap at the time of artificial urinary sphincter placement in patients at risk of cuff erosion. Int Urol Nephrol. 2020;52(5):851-57
- Peyronnet B, O’Connor E, Khavari R, Capon G, Manunta A, Allue M, Hascoet J, Nitti VW, Game X, Gilleran J, Castro-Sader L, Cornu JN, Waltregny D, Ahyai S, Chung E, Elliott DS, Fournier G, Brucker BM. AMS-800 artificial urinary sphincter in female patients with stress urinary incontinence: A systematic review. Neurourol Urodyn. 2018;38 Suppl 4:S28-41
- Chung E, Katz DJ, Love C. Adult male stress and urge urinary incontinence- A review of pathophysiology and treatment strategies for voiding dysfunction in men. Aust Fam Physician. 2017;46(9):611-666
- Chung E. A state of art review on the evolution of the urinary sphincter devices for the treatment of post-prostatectomy urinary incontinence: Past, present and future innovations. J Med Eng Technol. 2014;38(6):328-32
- Chung E and Cartmill R. Diagnostic challenges in the evaluation of persistent or recurrent urinary incontinence after artificial urinary sphincter (AUS) implantation in patients after prostatectomy. BJU Int. 2013;112( Suppl 2):32-35
- Chung E, Navaratnam A and Cartmill RA. Can artificial urinary sphincter be an effective salvage option in women following failed anti-incontinence surgery? Int UroGynae J Pelvic Floor Dysfunct. 2011;22(3):363-6
- Chung E and Cartmill RA. Twenty-five years’ experience in the outcome of artificial urinary sphincter in the treatment of female urinary incontinence. BJU Int. 2010;106(11):1664-7
- Chung E, Ranaweera M and Cartmill R. Newer and novel artificial urinary sphincters (AUS): The development of alternatives to the current AUS device. BJU Int. 2012;110(suppl4):5-11
- Chung E and Cartmill RA. The role of artificial urinary sphincter in female stress urinary incontinence. In: Sphincters: Properties, Types and Applications. Nova Science Publishers, Inc, NY
- Chung E. The American Medical System® artificial urinary sphincter (AMS 800): A historical perspective on the evolution in concept and design. In: Sphincters: Properties, Types and Applications. Nova Science Publishers, Inc, NY
- Chung E and Ranaweera M. A state of art review of current and future artificial urinary sphincters in the treatment of male urinary incontinence. In: Sphincters: Properties, Types and Applications. Nova Science Publishers, Inc, NY